Clinical Vignette: A Case of Nasal Aspergillosis
A one year and four-month-old male spayed cross-bred dog was presented for consultation with a history of monolateral (left sided) persistent epistaxis for the last 3 weeks. According to the owners the epistaxis has worsened in the last few days.
Physical examination revealed severe epistaxis from the left nostril. No other abnormalities were detected during the physical examination. The differentials diagnosis list for monolateral epistaxis include neoplasia, aspergillosis, foreign body and lymphoplasmacytic rhinitis. As the epistaxis was monolateral systemic disease such as coagulopathies (e.g. IMT), hypertension or hyperviscosity were considered less likely.
Haematology, biochemistry and electrolytes were unremarkable. Systolic non-invasive blood pressure was 130 mmHg. Aspergillus antibodies test was positive.
Under general anaesthesia a CT of the nasal cavity and frontal sinuses was performed (fig. 1-3). CT revealed marked lysis of the nasal turbinates in the left side of the nasal cavity, with some fluid accumulation. This lesion extended for about 8-10 cm aborally from the left nostril. The frontal bone was normal. There was dense fluid with gas pockets within the left frontal sinus.
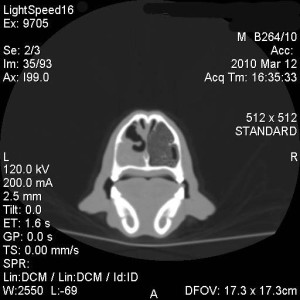
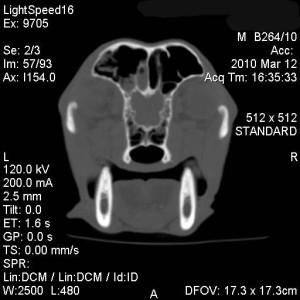
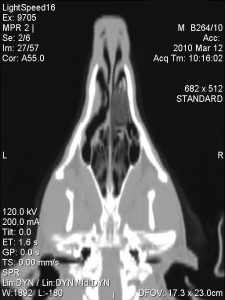
There was a mild amount of fluid in the rostral third of the right nasal cavities; no bone lysis was seen in this side.
A nasal endoscopy was performed. In the left side marked inflammation of the nasal mucosa with turbinates destruction was noticed, an abundant haemorrhage was also elicited during the procedure. In the right side there was a localised inflammation of the nasal mucosa with two yellow plaques on the surface of mucosa, only minor turbinate destruction was found
A retrograde nasal flush was performed in both nasal cavities. The fluid retrieved was analysed with cytology and culture. Bilateral nasal biopsies were performed. On the right side brush cytology and biopsies from the yellow plaques were taken.
Cytology revealed abundant neutrophilic inflammation with degenerate neutrophils, extra and intra cytoplasmatic cocchi were detected. Some septate iphae were detected from cytology of the plaques (fig. 4). Culture was positive Staphylococcus pseudointermedius sensitive to multiple antibiotics but negative for funghi.
Histology revealed severe ulcerative lymphocytic and neutrophilic inflammation with fungal forms (only in the right side) and bacterial forms.
The final diagnosis was nasal aspergillosis.
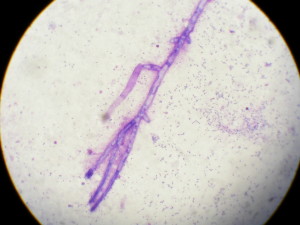
The diagnosis of nasal aspergillosis can be challenging in some situations. The test with the highest sensitivity is probably the identification of mucosal plaques with cytological or histological demonstration of fungal forms. However this is not always possible for several reasons. Commonly the severity of turbinate destruction, haemorrhages or inflammation do not allow a complete visualisation of the colonies in the sinuses, the fungal colonies are not infrequently localised aborally in the distal part of the turbinates or in frontal sinues, again not allowing a perfect visualisation. Combination of several tests as imaging and clinico-pathological findings may help to reach a diagnosis in most of the cases.
Advanced imaging such as CT or MRI give clear advantages over the traditional radiographs in term of detecting lesions in nasal cavity and frontal sinuses. The choice between CT and MRI relies mainly in their availability and clinician choice. CT provides better details of the bony structures and MRI has a better tissue contrast resolution and gives more information about soft tissue lesions.
Presence of antibodies against Aspergillus in serum has relatively low sensitivity (around 70%) but high specificity (around 98%), therefore cannot be use a sole test for a diagnosis but can be use as aid in the diagnosis in some cases.
Rhinoscopy is always necessary to identify nasal plaques and as an aid to take adequate samples from these lesions. Fungal culture and cytology are high sensitive tests only if the samples were taken directly from a fungal plaque or from a nasal biopsy, blind swabs or nasal secretion samples give frequently false negative result in case of Aspergillosis.
The dog was treated with flush of clotrimazole liquid 1% in the nasal cavities and frontal sinuses after trephination of the frontal sinuses followed by instillation of clotrimazole cream as depot agent, this treatment was repeated one month after.
Broad-spectrum antibiotic, selected accordingly to the cultural sensitivity, was also given for 3 weeks after the diagnosis for secondary bacterial infection.
Different treatments for nasal aspergillosis have been published in the literature. None of the published studies showed 100% efficacy or included a relevant number of cases allowing to establish a clear better treatment compared to the others. Clinician experience, patients and owners compliances play a significant role in the decision-making process which technique to use.
We opted for clotrimazole depot technique as it does not need a long hospitalisation, it has relatively high percentage of success rate (around 80%) and he is relatively less stressful for the patient.
Two weeks after the first treatment the dog has stopped any nasal discharge and was doing clinically very well.
The long-term prognosis for nasal aspergillosis is fair. Recurrence is not uncommon and it varies according the studies from 10% to 35%. This can happen from few weeks until four years after the treatment. New course of treatment is advised after a new diagnostic work-up as chronic rhinitis (not fungal) and few cases of nasal neoplasia have been described after treatment of nasal aspergillosis.

