Medical Treatment of Infectious Otitis Externa and Media
Abstract
Otitis externa, inflammation of the external ear canal, with or without middle ear involvement, is very common in dogs and quite common in cats. Ear disease is seen in both first opinion and referral practice and due to its frequently recurrent nature, it constitutes a frustrating problem for owners and veterinarians alike. Many different factors can cause or exacerbate otitis, and recognition and correction of these is the key to successful management. Based on history and clinical presentation, management of ear diseases include medical and surgical options. The aim of these notes is to describe the medical interventions available for otitis externa and media, discussing the principles of ear cleaning, ear flushing, and use of topical and systemic medications, mentioning the issues related to ototoxicity.
Medical treatment of infectious otitis externa and media
Once an otic infection has been diagnosed, medical options include topical and systemic therapy, co-adjuvated by ear cleaning, when appropriate.
Ear cleaning – when?
Whilst it is commonly accepted that cleaning is not necessary in healthy ears, it is beneficial in the following conditions:
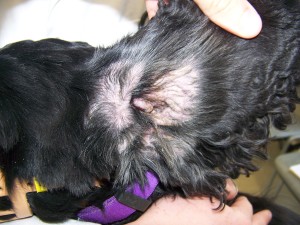
- Seborrheic ears (Fig. 1)
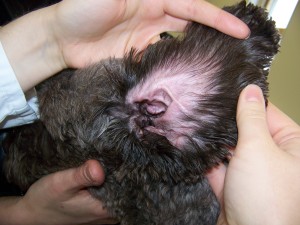
- Hairy ears (Fig. 2)
- Stenotic ears
- Pendulous ears
- Purulent discharge
Ear cleaning is an important part of any treatment regimen as it can remove debris and pus and permit complete diagnostic evaluation of the ear canal and tympanic membrane.
The cleaning fluids most commonly contain:
- Ceruminolytics, surfactants and foaming agents e.g. sodium docusate
- Astringents or drying agents e.g. isopropyl alcohol
- Antimicrobial agents e.g. parachlorometaxylenol (PCMX)
In a study (Swinney et al. 2008) the antimicrobial efficacy of different ear cleaners against Staphylococcus intermedius, Pseudomonas aeruginosa and Malassezia pachydermatis was evaluated. Antimicrobial activity appeared to be associated with the presence of isopropyl alcohol, parachlorometaxylenol and a low pH. Manual cleansing can be done at home by the owners; however it is important to instruct them on how to perform the cleaning and how often to use the different preparations. Although these are normally not recommended to be used more than every 48h, in one study(Cole et al. 2003) one cleaner (EpiOtic, Virbac) used up to twice daily caused no adverse effects. Manual cleansing doesn’t remove tightly adherent debris or material present in the deep portion of the ear canal and therefore is best used as routine cleansing at home once ear flushing has been performed.
You might also be interested in:
Ear flushing
Ear flushing is indicated when the entire external ear canal and/or the middle ear need thoroughly cleaning. It should always be performed under general anaesthesia with an endotracheal tube placed and cuffed, to avoid the fluids running from the ear to the respiratory tract through the Eustachian tube. In the presence of hyperplastic, stenotic or particularly inflamed ear canals, it is recommended to provide systemic glucocorticoid treatment (0.5 – 1mg/Kg once daily) 2-3 weeks prior to the flushing. Ear flushing is best to be performed by using a video-otoscope or, if not available, with a urinary catheter or a feeding tube connected to a syringe and fluids (sterile saline), preferably through a three-way tap. Before ear flushing is performed, some cases may require the use of an ear cleansing solution to emulsify and remove debris. If the ear drum cannot be visualised, care should be taken as ear cleaners are not licensed for applications in the middle ear and are all potentially ototoxic.
Myringotomy
Iatrogenic rupture of the tympanic membrane is indicated when otitis media is suspected and/or confirmed by diagnostic imaging techniques, to take samples for cytology and culture from the tympanic bulla and to allow flushing of the middle ear cavities. It should be performed under general anaesthesia and under direct visualisation after lavage of the external ear when the canal is dry. The preferred method used by the author is using a 6 French urinary catheter cut obliquely to a 60° and attached to a 2ml syringe containing sterile saline solution. The catheter is advanced through the ventral and posterior quadrant of the membrane with subsequent aspiration of the fluids. An aliquot can be used for direct cytological examination and the remainder for culture.

Topical Therapy
In the majority of cases of infectious otitis externa, topical therapy alone is sufficient.
Antimicrobial agents rarely can reach, systemically, therapeutic concentrations in the skin of the ear canal and topical therapy, chosen empirically based on otic cytology and otoscopic examination, represents an appropriate choice. Antibiotic sensitivity data reflects the serum level needed systemically, and it is less useful with topical drugs where concentrations 100 to 1000 times superior to the MIC (Minimum Inhibitory Concentration) may be reached. Topical therapy usually is characterised by high efficacy and, with regards to the antimicrobial agents, by no systemic side effects in the presence of an intact tympanic membrane.
Ingredients of topical antibacterials
Fusidic Acid
- Bacteriostatic
- Effective agaisnt Gram positive cocci
- Mechanism of action: interference with bacterial proteins synthesis
Aminohlycosides
- Bactericidal
- Large spectrum
- Mechanism of action: interference with bacterial proteins synthesis
- Impaired in an acidic environment – cleansing agents should be used at least one hour prior to their use
- Topical agents include mainly neomycin and gentamicin. Amikacin is not available as topical preparations, but injectable formulations can be used
- Topically, when diluted in sterile saline
Polimixin B
- Bactericidal
- Effective against Gram negative bacteria
- Mechanism of action: alteration of cytoplasmic membrane permeability
- Ototoxic
- Inactivated by cellular debris, therefore the association with ear cleaning is important
Fluoroquinolones
- Bactericidal
- Large spectrum
- Mechanism of action: inhibition of DNA replication
- Effective against Gram positive and Gram negative bacteria
- Topical agents, available as veterinary formulations, include marbofloxacin and orbifloxacin. Enrofloxacin injectable solution, diluted in sterile
- saline, can be used topically
Carboxypenicillins
Expanded-spectrum penicillins
Active against gram-negative organisms (including Pseudomonas spp)
Mechanism of action: penetrates the gram-negative cell membrane
Reconstituted ticarcillin, diluted with sterile water, is the carboxycillin for which topical use has been most commonly reported in the treatment of canine Pseudomonas otitis
Topical Antimycotics
Azoles
- Amidazoles (clotrimazole, miconazole, posaconazole and ketoconazole)
– Mechanism of action: inhibition of ergosterole synthesis
Polienic
- Nistatine
– Mechanism of action: binds to ergosterole causing alterations of the cellular wall permeability
Other topical antimicrobials
- Silver Sulfadiazine (SSD) – this has broad-spectrum antibacterial activity (most notably against P. aeruginosa). Concentrations as low as 0.02% have shown 100% efficacy against P. aeruginosaandStaphylococcus spp. It is available as a cream and, although not readily miscible in water, a homogeneous emulsion can be achieved with gentle mixing.
- Tromethamine-ethylenediamine-tetraacetate (TrisEDTA) –this is commonly used as either a pre-soak or a carrier vehicle in the treatment of gram negative infections. EDTA promotes increased permeability to extracellular solutes and increased sensitisation to antibiotics, whereas Tris serves as a buffer. Recently (Guardabassi et al. 2010) the in vitro antimicrobial activity of a commercial ear antiseptic containing chlorhexidine (0.15%) and Tris-EDTA (Otodine Vetruus) was evaluated; according to the results, this product was active against all the pathogens most commonly involved in canine otitis.
- Bacteriophage treatment. A recent publication (Hawkins et al. 2010) reported the results of a veterinary clinical trial of a bacteriophage treatment of infection. The results show that administration of this topical bacteriophage mixture leads to lysis of P. aeruginosa in the ear without apparent toxicity.
Systematic Therapy
Antibiotics
As stated by Morris (1994)“Unless the ear canal epithelium has been eroded or ulcerated extensively, systemic (oral) antimicrobials are unlikely to achieve therapeutic concentrations within the fluid and waxy exudates ofthe external canals in which the infectious organisms are harboured.”
Considering that the middle ear (tympanic bulla) contains a highly vascular mucous membrane lining, drugs may diffuse from the vascular compartment to the bulla space better than in the external ear canals. The choice of systemic antibiotics for treating the middle ear diseases is also indicated as the tympanic bulla may present a problematic access to topicals. The choice should be based on culture and susceptibility testing and, results pending, empirical treatment, based on examination of cytological specimen from the bulla content, should be started.
Antibiotics recognised effective for the treatment of otitis media include:
- Enrofloxacin 5-20mg/Kg once daily
- Marbofloxacin 2-5mg/Kg once daily
- Ciprofloxacin (off label) 10-20mg/Kg once daily
- Orbifloxacin 2.5mg/Kg once daily
- Cefalexin 20-30mg/Kg twice daily
In case of multi-resistance:
- Ceftazidime (off label) 30mg/Kg four times daily
- Ticarcillin (off label) 40-80mg/Kg three times daily
- Meropem (off label) 8mg/Kg twice daily
Antimicotics
The administration of systemic antimicotic agents is needed in cases of otitis media caused by Malassezia spp. or when topical therapy is not an option.
Drugs used include:
- Ketoconazole (off label) 10mg/Kg once daily
- Itraconazole (off label) 5mg/Kg once daily
- Fluconazole (off label) 2.5-5mg/Kg once daily
Glucocorticoids
Administered systemically they can:
- Reduce stenosis
- Reduce oedema
- Reduce hyperplasia
- Allow otoscopic examination
- Allow a better cleaning process
The initial treatment consists of doses of 0.5-1mg/Kg once daily, depending on the severity of clinical signs. Dose and frequency of administration should be reduction until discontinuation when the medication is no longer needed.
You might also be interested in:
Ear wicks
Ear wicks are made of polyvinyl alcohol (PVA) and are characterised by a hard compact structure. They are inserted into the ear canal under general anaesthesia and then soaked with a solution usually containing antibiotics with or without TrizEDTA and/or glucocorticoids. The expansion produces a structure that adapts to the contours of the ear canal, slowly releasing the medicaments. In this author’s experience, ear wicks can be a useful alternative to daily topical therapy in those patients that do not tolerate administration of topical medications. It is paramount that the ear canals and (in the presence of otitis media) the tympanic bullae are aseptically cleaned prior to the placement, as if the canal is not adequately flushed, the wick can act as a lid trapping infections. Additionally, in dogs with large ear canals, they often do not expand sufficiently to fill the ear canal in its entire diameter.
Ototoxicity
An ototoxic agent can cause damage to the ear in any of its anatomical components. Usually ototoxicity can be divided: cochlear damage with consequent deafness or vestibular damage with consequent vestibular syndrome. In both cases, the damage occurs to the inner ear. The ototoxic agent can reach the inner ear via haematogenous route or directly through openings in the tympanic membrane. In particular, given that the middle and inner ear component can be damaged by topical medicaments, it is important that before administration of a topical, the clinician performs an otoscopic examination.
Systemic ototoxicity
Examples of molecules that cause ototoxicity after topical administration are aminoglycosides antibiotics, furosemide, cisplatin, vinblastin, vincristin.
Local ototoxicity
Cleaning agents. According to Mansfield et al. (1997)the only non ototoxic agent is squalene. With regards to chlorhexidine, recent studies have demonstrated that if used at a 0.2% or inferior concentration, it doesn’t carry a risk of ototoxicity.
Antibiotics. Aminoglycosides can cause cochlear damage, with the exclusion of gentamicin that in a study(Strain et al. 1995) failed to cause damage when instilled in the middle ear of dogs for 21 days. Agents with recognised ototoxic potential are polimixin B in the guinea pig and ticarcillin in the chinchilla.
Agents with low ototoxic potential are fluoroquinolones, somecefalosporines (e.g. ceftazidime), the antifungals clotrimazole, miconazole, nistatine and tolfaftate, the steroids desamethasone and fluocinolone, and TrisEDTA solution.
In pathologic condition, even the use of simple saline solution during ear flushing may cause, although infrequently, complications. For this reason it is always important to inform owners about potential risks of ear flushing and topical therapy.
Finally, it is paramount to highlight that the majority of the studies on ototoxicity have been performed on laboratory animals or have been extrapolated by human studies. Despite these studies representing guidelines for the clinician, further studies on other species are needed.
References
1) Cole L.K. Treatment of infectious otitis externa and media. Proceedings of 6th World of Veterinary Dermatology: 95-99.
2) Cole L.K., Kwochka KW, Kowalski JJ, Hillier A, Hoshaw-Woodard SL. Evaluation of an ear cleanser for the treatment of infectious otitis externa in dogs.Vet Ther. 2003 Spring;4(1):12-23
3) Cole L.K., Papich M.G., Kwochka K.W., Hillier A., Smeak D.D., Lehman A.M. Plasma and ear tissue concentrations of enrofloxacin and its metabolite ciprofloxacin in dogs with chronic end-stage otitis externa after intravenous administration of enrofloxacin.Vet Dermatol.2009: 20(1): 51-9.
4) Gotthelf L.N. Diagnosis and treatment of otitis media in dogs and cats.Vet Clin North Am Small AnimPract. 2004: 34(2):469-87.
5) Gotthelf L.N. Ototoxicity in: Small Animal Ear Diseases: An illustrate Guide. 2nd Edition W.B. Saunders Company, Philadelphia, 2005: 329-349.
6) Graham-Mize C.A., Rosser E.J. Jr. Comparison of microbial isolates and susceptibility patterns from the external ear canal of dogs with otitis externa. J Am AnimHosp Assoc. 2004: 40(2):102-8.
7) Harvey R.G., Harari J., Delauche A.J. Ototoxicity and other side-effects of otic medication. In: Ear Diseases of the Dog and the Cat. Iowa State Press 2005: 213-218.
8) Kiss G., Radvanyi S.Z., Szigeti G. New combination for the therapy of canine otitis externa.I: microbiology of otitis externa. J Small AnimPract 1997: 38: 51-6.
9) Logas D. Appropriate use of glucocorticoids in otitis externa. In: J.D. Bonagura (editor) Kirk’s Current Veterinary Therapy XIII. Small Animal Practice. W.B. Saunders, Philadelphia, 2000: 585-586.
10) Lorenzini R., Mercantini R., De Bernardis F. In vitro sensitivity of Malassezia spp. to
various antimycotics. Drugs ExpClin Res 1985: 11(6): 393-5.
11) Mansfield P.D., Steiss J.E., Boosinger T.R., et al. The effects of four commercial ceruminolytics on the middle ear. J Am Hosp Assoc 1997: 33: 479-486.
12) Merchant S.R, Neer T.M., Tedford B.L., et al. Ototoxicity assessment of a chlorhexidine otic preparation in dogs. Prog Vet Neurol 1993: 4: 72-75.
13) Morris D.O. Medical therapy of otitis externa and otitis media.Vet Clin North Am Small AnimPract.2004: 34(2):541-55.
14) Nuttall T., Cole L.K. Evidence-based veterinary dermatology: a systematic review of interventions for treatment of Pseudomonas otitis in dogs.Vet Dermatol. 2007: 18(2): 69-77.
15) Pinchbeck L.R., Hillier A, Kowalski J.J., Kwochka K.W. Comparison of pulse administration versus once daily administration of itraconazole for the treatment of Malasseziapachydermatis dermatitis and otitis in dogs. J Am Vet Med Assoc 2002: 220: 1807-12.
16) Guardabassi L, Ghibaudo G, Damborg P. In vitro antimicrobial activity of a commercial ear antiseptic containing chlorhexidine and Tris-EDTA.Vet Dermatol. 2010 Jun;21(3):282-6.
17) Hawkins C, Harper D, Burch D, Anggård E, Soothill J.Topical treatment of Pseudomonas aeruginosa otitis of dogs with a bacteriophage mixture: a before/after clinical trial.Vet Microbiol. 2010 Dec 15;146(3-4):309-13.
18) Strain G.M., Merchant S.R., Neer T.M., et al. Ototoxicity of a gentamycin sulphate otic preparation in dogs. Am J Vet Res 1995: 532-538.
19) Tom L.W. Ototoxicity of common topical antimycotic preparations. Laryngoscope 2000:110: 509-16.